ASCO’s Media Issue Briefs provide succinct overviews and relevant data on major policy issues impacting patients with cancer and the physicians who care for them. These briefs are designed to be especially helpful for journalists, offering background information on key issues across health policy today. Access ASCO’s full collection of Media Issue Briefs.
Issue Overview
Eligibility criteria are the specifications that determine who is eligible to participate in a clinical trial. Outlined in the study protocol, criteria may be inclusive or exclusive based on characteristics such as type and stage of disease, previous treatment history, age, and other medical or non-medical attributes.
In addition to defining the population of a clinical trial, eligibility criteria are intended to protect the safety of trial participants – particularly those populations that may be more vulnerable to adverse events of a trial drug. Overly restrictive eligibility criteria, however, can harm patient accrual for clinical trials; restrict patient access to investigational drugs; contribute to increased trial complexity, length, and cost; and limit the generalizability of study results to the broader population of patients who will ultimately use the drug.
Key Data
Common Exclusion Criteria
Common exclusion criteria have developed over time and grown in complexity with clinical trials.1 A study of 21 cancer trials found an average of 16 eligibility criteria per trial with 60% related to comorbidity or performance status.2,3
An analysis of Investigational New Drug applications for 2015 found that, of 250 study protocols:
- Only five (1.7%) allowed enrollment of HIV-positive patients with stable disease and/or adequate CD4+ T-cell counts.
- Less than half (140 or 47.1%) allowed participation of patients with treated or stable brain metastases.
- Only 11 protocols (3.7%) included pediatric patients younger than 16 years of age.4
Some have suggested that eligibility criteria often are adapted from a past protocol for a similar study, but should instead be determined based on the specific biological mechanisms and safety profile of the investigational drug.5
Cancer Clinical Trial Accrual
Patient accrual for cancer trials continues to be a challenge in the United States.
- Approximately 3% of U.S. adult patients with cancer enroll in a clinical trial.6
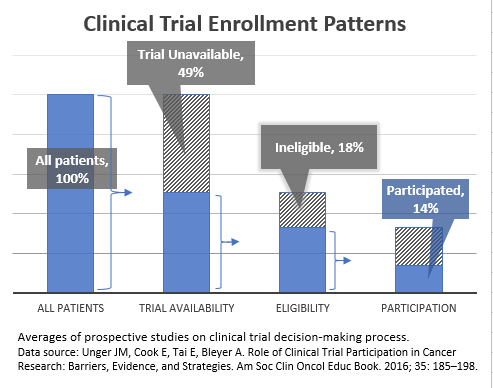
- Nearly one in five (18%) publicly funded cancer studies are unable to recruit enough participants.7 Closing trials due to low accrual effectively ends the research before conclusions can be reached, wasting substantial time and resources invested to launch the clinical trial.
Restrictive eligibility criteria can create barriers for patients to access clinical trials and may contribute to prolonging the accrual process or early closure of studies struggling with patient accrual.
- An analysis of cancer studies found that ineligibility was the cause of 18% of patients with cancer not participating in a clinical trial (Figure 1).2
Generalizability of Trial Results
Limited safety and efficacy data may be available to inform treatment of patient populations who are excluded from trial participation and generally older and less healthy than clinical trial participants. This can include patients who may make up a significant portion of the intended-use population of a drug following approval. For example, since brain metastases are more common in some cancer patient populations, the frequent exclusion of patients with active, stable, or treated brain metastases may mean that 50%-75% of intended-use populations are left out of findings for a drug’s efficacy and safety.8
Eligibility Criteria in the Molecular Age
The growth in molecularly targeted cancer treatments creates a need and opportunity to create more inclusive eligibility criteria to continue the pace of research progress. Many common eligibility criteria were developed based on traditional chemotherapy treatments, with very different toxicity profiles than molecularly driven therapies. The increase in investigative targeted therapies may open the door for the safe expansion of eligibility to certain patient populations in some cases.1
Additionally, restrictive eligibility criteria can have a more pronounced effect on trial accrual in the context of targeted therapies. Molecularly targeted drugs rely on distinctive patient biomarkers to treat cancer that may only be applicable to a very small pool of cancer patients. This is exemplified in non–small-cell lung cancer (NSCLC) where marker incidence among patients can be as low as 1% to 6%,9 despite the prevalence of the disease.1 If other eligibility criteria can be expanded, it will help more patients with the biomarkers qualify for the targeted therapy trials.
ASCO-Friends of Cancer Research Broadening Eligibility Criteria Project
ASCO and Friends of Cancer Research launched a joint project in 2016 to promote more inclusive eligibility criteria in cancer clinical trials. The organizations identified areas where eligibility criteria were most likely to restrict a patient’s participation in a trial, but least likely to affect the safety of participants. Working groups, comprised of researchers, patient advocates, regulators, and industry representatives, developed comprehensive recommendations to address eligibility criteria in five areas: minimum age requirements for trial enrollment, HIV/AIDS status, brain metastases, organ dysfunction, and prior and concurrent malignancies.
Articles developed by each of the working groups were published, along with an ASCO-Friends research statement, as a Journal of Clinical Oncology Special Series in October 2017. ASCO and Friends are working with clinical trial sponsors and regulators to implement these recommendations in protocols and identify additional opportunities to safely expand eligibility criteria for oncology trials.
More Information
- Broadening Eligibility Criteria for Clinical Oncology Trials Journal of Clinical Oncology Special Series
- ASCO-Friends of Cancer Research Joint Research Statement: Broadening Eligibility Criteria to Make Clinical Trials More Representative
- FDA Analysis of Investigational New Drug Applications in 2015: Re-Evaluating Clinical Trial Eligibility Criteria
- ASCO in Action offers the latest news and information on this and other cancer policy topics.
- To schedule a media interview with an ASCO spokesperson or oncology expert, please contact mediateam@asco.org.
1. Kim ES, Bernstein D, Hilsenbeck SG, et al. Modernizing Eligibility Criteria for Molecularly Driven Trials. J Clin Oncol. 2015; 33:25, 2815-2820.
2. Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106:dju002.
3. Unger JM, Cook E, Tai E, Bleyer A. Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am Soc Clin Oncol Educ Book. 2016; 35: 185–198.
4. Jin S, Pazdur R, Sridhara R. Re-evaluating eligibility criteria for oncology clinical trials: analysis of investigational new drug applications in 2015. J Clin Oncol. 2017;35(33):3745–3752.
5. Kim ED, Bruinooge SS, Roberts S, et al. Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol. 2017; 35:33, 3737-3744.
6. Institute of Medicine Forum on Drug Discovery, Development, and Translation: Transforming clinical research in the United States: Challenges and opportunities: Workshop summary.
7. Bennette CS, Ramsey SD, McDermott CL, et al: Predicting low accrual in the National Cancer Institute’s Cooperative Group clinical trials. J Natl Cancer Inst 108: djv324, 2015.
8. Lin NU, Prowell T, Tan RA, et al: Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology–Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol. 2017;35(33):3760-3773, 2017.
9. GJ Korpanty, DM Graham, MD Vincent, et al: Biomarkers that currently affect clinical practice in lung cancer: EGFR, ALK, MET, ROS-1, and KRAS. Front Oncol 4: 204,2014











